Eyal Orion
Founder & CEO
The invitation to claim the profile was originally sent to
The link to share this saved search was copied to your clipboard.
Paste it where you want to share.
Also shared
Also shared

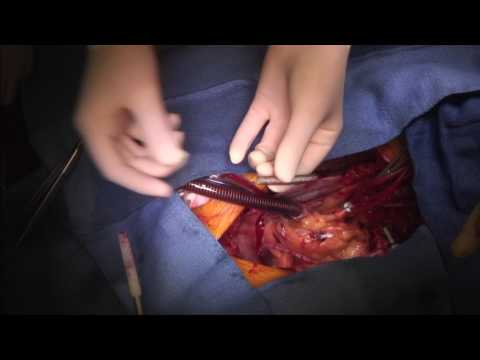

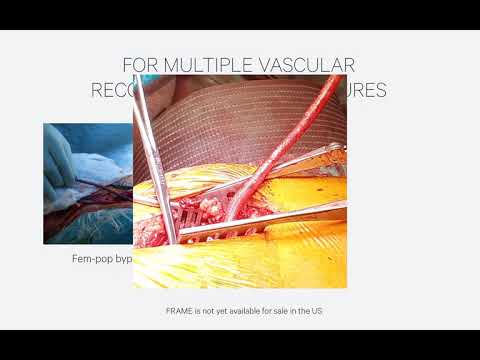

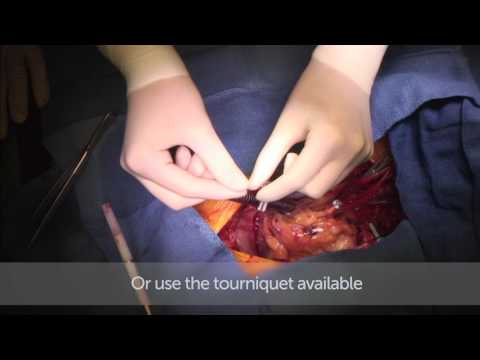

Also shared
Also shared